- ISO 15189
Medical Laboratory Accreditation
Providing confidence that medical laboratories deliver quality levels of performance and competence.
Accreditation for healthcare professionals
-
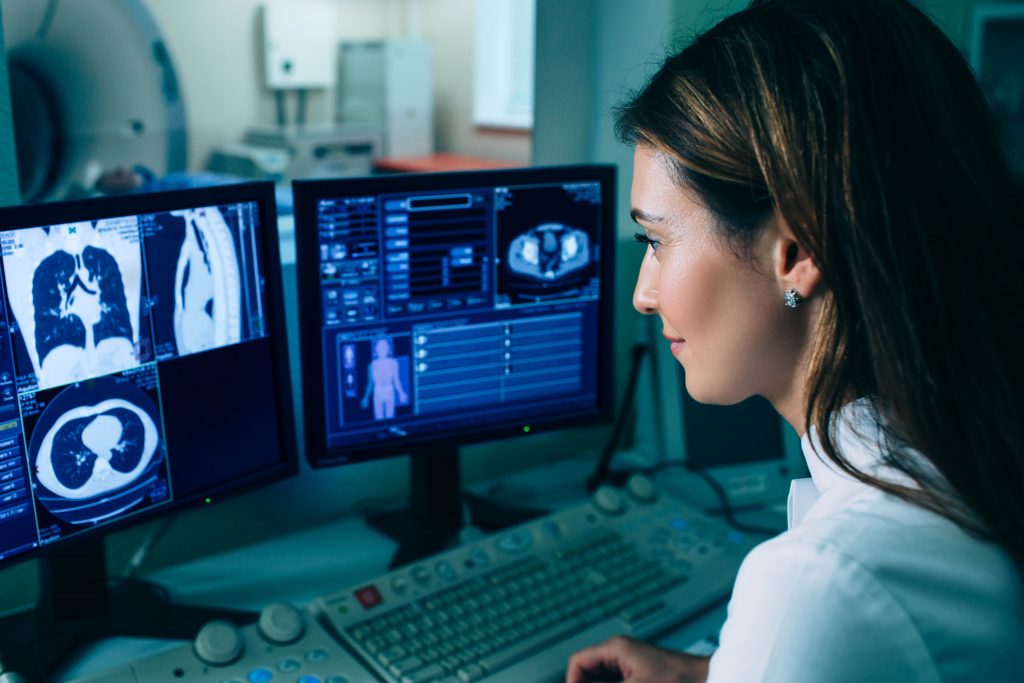
Accreditation for diagnostic medical imaging to BS 70000
UKAS accreditation of imaging services is a patient-focused assessment that is designed to help diagnostic imaging services ensure that their patients consistently receive high quality services, delivered by competent staff working in safe environments.
-
Improving Quality in Physiological Services (IQIPS)
UKAS supports the delivery of quality physiological services based on a formal and impartial assurance process designed to deliver clearly defined and professionally led standards.
-
Medical Physics & Clinical Engineering (MPACE) to BS 70000
Accreditation underpins the delivery of safe, accurate and expert procedures and practices relating to medical physics and clinical engineering. It also provides assurance that equipment is managed, maintained and operated to recognised quality standards
-
Accreditation of Proficiency Testing to ISO/IEC 17043
Accreditation for Proficiency Testing (PT) scheme providers to demonstrate their competence to plan and implement proficiency testing programs.